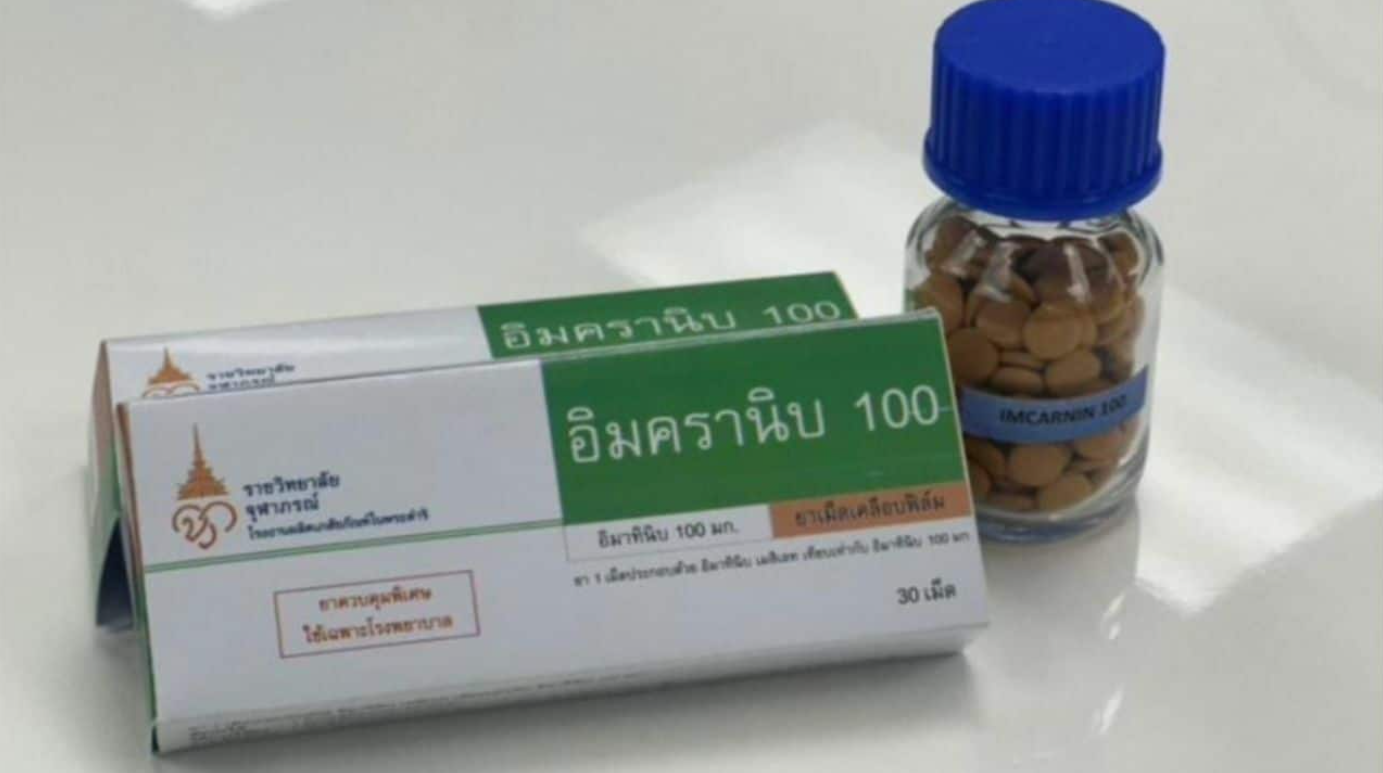
Thailand has taken a bold leap in healthcare innovation. On May 20, 2025, the Thai FDA approved Imcranib 100, the nation’s first domestically produced targeted cancer tablet containing 100 mg of imatinib. Unlike traditional chemo, which hits everywhere, this pill zooms in on cancer cells—helping patients stay stronger and feel better.
What Makes Imcranib 100 Special?
This isn’t just any generic drug—it’s a tyrosine kinase inhibitor (TKI). In simple terms? It jams the enzyme signals that turbocharge cancer cell growth. What’s awesome by itself: It’s Thai-made, meaning:
-
Lower costs—no more sky-high import bills.
-
Easier access—waived restrictions for more patients.
-
National pride—homegrown excellence, from lab to shelf.
Who Can It Help?
| Cancer Type | Benefit |
|---|---|
| Chronic Myeloid Leukaemia (CML) | Better disease control |
| Ph+ Acute Lymphoblastic Leukaemia (Ph+ ALL) | Precise molecular targeting |
| Gastrointestinal Stromal Tumours (GIST) | Slows tumour growth |
| Dermatofibrosarcoma Protuberans (DFSP) | Rare skin cancer option |
This milestone was driven by Her Royal Highness Princess Chulabhorn Krom Phra Srisavangavadhana, who has championed cancer research and treatment access. A government-run plant in Chonburi—Thailand’s first GMDP PIC/s certified—produces Imcranib. The expert team at Chulabhorn Hospital is now piloting distribution with full safety tracking, drug-management protocols, and solid clinical oversight .
Why This Is a Big Deal
-
Cut Costs & Boost Equity
No longer forced to import expensive medicines—cutting costs and making treatment fairer for more people. -
Tech & Talent Growth
Thailand’s pharm‑tech muscles just got bigger. From research labs to regulatory processes, capabilities just leveled up. -
Future Drug Pipeline
The factory and experts pave the way for future breakthroughs—think biologics and immunotherapies coming from Thai R&D .
What’s Next?
-
July 2025 marks the official pilot rollout at Chulabhorn Hospital, with clinical systems to monitor effectiveness and safety.
-
Expect insurance policy updates, lifting coverage limits for all FDA‑approved uses.
-
A wider wave of clinical trials, local drug formulation, and biotech investment to come.
Conclusion
Thailand’s launch of Imcranib 100 isn’t just a pill—it’s a statement. It says: We can innovate, we can produce, we can care for our people without relying on imports. It’s a lifeline for cancer patients—and a ladder into a future where Thailand isn’t just a drug consumer, but a drug creator.